It almost seems like science fiction: inside every human body, fierce battles are raging, invisible to the naked eye. Your immune system throws everything it’s got at invaders, while bacteria deploy elaborate tricks to avoid defeat. Some of these microscopic villains have been evolving their sabotage skills since before dinosaurs showed up, and they’re not giving up now. Why does this matter? Because the way these tiny battles play out decides who gets sick, how diseases spread, and even how vulnerabilities pass from one person to the next.
The Immune System’s First Defense: Cells on Patrol
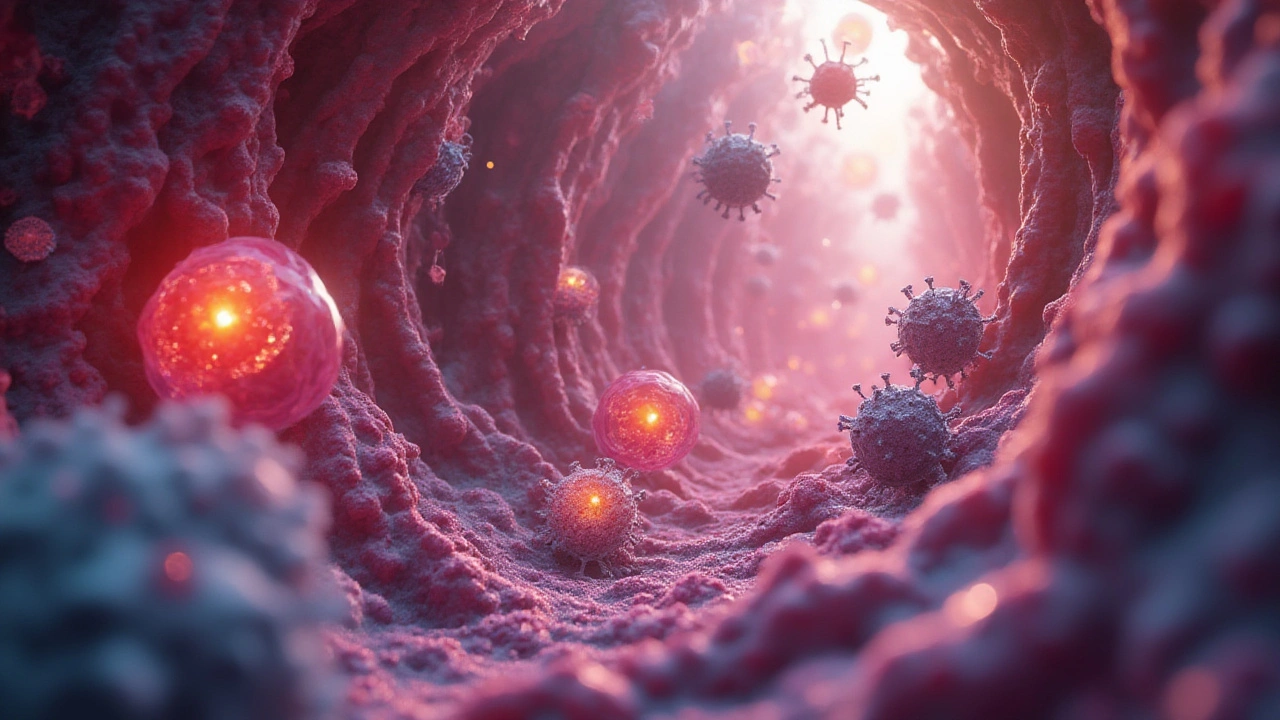
Think of your immune system as an organized city guard. You don’t notice them, they’re always on duty, and their focus is to keep invaders out. The very first line of defense is physical—your skin, mucous membranes, and sneezing reflex. But as soon as a bacterium slips past these guards, things get real. Inside your body, white blood cells are constantly circulating, looking for anything suspicious. Macrophages are one of the stars of the show. They sniff out invaders, engulf them, and blast them with powerful enzymes. Neutrophils, meanwhile, act fast and furious — they’re the immune system’s rapid response team.
Here’s the thing: not all bacteria are equal. While most are harmless (or even helpful—more on that later), a few bring serious trouble. When these bad actors, or pathogens, crash the cell’s party, they’ll meet a series of detection checkpoints. Dendritic cells notice odd patterns on the bacteria’s surface (known as PAMPs—microbe fingerprints) and send out alarm signals. This sparks an inflammatory response, recruiting more immune cells and increasing local temperature—yes, that’s what a fever is about.
What’s truly wild? Recent research shows some bacteria can actually block the host’s molecular alarms. For example, the notorious Salmonella can inject special proteins (using a little syringe-like tool) that silence early warning signals inside host cells. In one 2024 survey at the University of Toronto, scientists found that some strains of E. coli altered their surface so they could slip past initial detection completely. It’s like they showed up wearing a disguise.
Bacterial Tricks: How Pathogens Persist and Escape
Bacteria aren’t passive; they’re masterminds at survival. Picture them as clever thieves. The list of their tricks is always growing, thanks to discoveries in cell biology and genomics.
- Immune Evasion: Many bacteria coat themselves with slippery sugars or proteins to dodge immune attacks. Strep bacteria, for instance, uses a layer called a capsule—kind of like wearing a raincoat to avoid being grabbed.
- Hijacking Host Processes: Some bacteria slip into cells and rewire them from inside, much like a hacker gaining access to a secure server. Listeria can ride along the inner skeleton of a cell, pushing and poking to escape to the next one without ever being exposed to the outside.
- Molecular Sabotage: Certain toxins produced by pathogens destroy barriers or scramble cell signaling. Take Staphylococcus aureus: its toxins rupture host cell membranes, creating chaos and confusion for immune defenders.
- Genetic Flexibility: Bacteria swap genes like collectors trade baseball cards. Horizontal gene transfer means antibiotic resistance or new camouflage tricks can spread overnight through a whole population.
Some of these tactics mean a single cell can spark a deadly infection if your immune system loses step. In Halifax’s own Queen Elizabeth II Health Centre, clinicians recently documented a cluster of infections where MRSA (methicillin-resistant Staphylococcus aureus) sidestepped common antibiotics and went straight for deeper tissue—a reminder that these battles aren’t abstract, but happening in real clinics right now.

The Host Fights Back: Weaponizing Cell Defenses
Your cells aren’t helpless — far from it. Once they recognize invaders, they unleash a biotech arsenal. Cells produce reactive oxygen species (a kind of chemical grenade) to blast bacteria caught inside. Others, called natural killer cells, hunt down infected cells and quietly destroy them before they can become a problem.
One of science’s neatest revelations was the discovery of CRISPR, a genetic cut-and-paste tool that bacteria themselves use as an immune memory. They keep molecular mugshots of past invaders (usually viruses), allowing them to slice up any familiar threats that return. Strangely enough, researchers at Dalhousie University in spring 2025 showed that pathogenic bacteria sometimes use a version of CRISPR to fight off helpful elements in their environment, making them tougher and more persistent than ever.
But the real arms race? It’s the adaptive immune response—B and T cells. After the body’s first defense line, these cells get involved, searching for unique proteins on the surface of bacteria and producing custom-shaped antibodies. These antibodies can find bacteria in circulation and flag them for destruction.
Not every defense is perfect, of course. Autoimmune diseases happen when the immune system mistakes your own cells for invaders. Sometimes bacteria exploit these mistakes, tricking cells into harming the body. Understanding these maneuvers helps doctors create vaccines or therapies that train your immune system to be smarter, not just stronger.
Case Studies: Diseases Shaped by Host–Pathogen Battles
So, what happens when things go sideways? Take tuberculosis — an age-old disease making a comeback in parts of Canada. The culprit, Mycobacterium tuberculosis, can hide inside immune cells for years, walling itself off in a fortress built from host tissues (called granulomas). In many cases, the body never quite clears the infection, and under stress it can resurface, causing dangerous chronic illness. That “hide-and-seek” is a classic host–pathogen arms race in action.
Here’s a quick look at some diseases and the strategies involved:
| Disease | Bacterial Tactic | Host Response |
|---|---|---|
| Pneumonia (Streptococcus pneumoniae) | Capsule evasion, toxin production | Antibody response, inflammation, fever |
| Urinary tract infection (E. coli) | Pili to stick to cell linings | Sloughing off infected cells, neutrophil influx |
| MRSA infections | Antibiotic resistance, biofilm formation | Adaptive immunity, targeted antibiotics, phage therapy |
| Cholera (Vibrio cholerae) | Toxin triggers water loss from cells | Rapid immune activation, dehydration management |
Real-world data also tells us some infections are harder to fight off if you’re run-down. Stress, poor nutrition, or even lack of sleep impact your cell defenses. Halifax’s Public Health Agency reported a spike in bronchitis cases last winter, and most were linked to immune system fatigue in the aftermath of the flu. It’s a reminder that your everyday choices can tilt the scale in these microscopic wars.

What’s Next: New Findings and Everyday Impacts
Science isn’t standing still. Right now, research is pushing the boundaries on host–pathogen interactions, with surprising results. For instance, a 2025 McGill University study detailed how certain probiotics coax the immune system to “learn” faster from bacterial battles, leading to fewer repeat infections. Other labs are developing therapies that mimic immune tactics—think molecular decoys or custom antibodies—to disarm bacteria before they cause damage.
If you’re wondering how this science could actually affect your daily life, consider these tips:
- Keep your microbiome in shape. Eating fiber-rich foods encourages the growth of helpful bacteria that can crowd out bad actors.
- Stay up to date on vaccines. Vaccination gives your adaptive immune system a head start by introducing safe “practice” targets.
- Don’t misuse antibiotics. Taking antibiotics for non-bacterial infections (like colds) helps bacteria evolve resistance faster.
- Prioritize sleep, stress management, and regular exercise—your immune cells work better when you do.
- Wash your hands often, especially after contact with public surfaces—fewer invaders in means fewer battles to fight.
Curious about just how bacteria launch their sneak attacks, or which methods help you bounce back faster? Check out these pathogenic bacteria insights for a deeper look at what’s lurking under your microscope.
Every person’s cells are playing defense right now, maybe even as you read this. Sometimes I wonder what Phlox, my cat, is fighting off between naps on the windowsill—turns out, animals have their own version of these cellular battles. Whether you’re thinking about the flu, food poisoning, or just a weird tickle in your throat, it’s all part of the unseen war that shapes your health. The more we learn about host–pathogen interactions, the closer we come to giving our bodies the fighting edge—every single day.







15 Comments
Courtney Mintenko
July 22, 2025 AT 20:09So bacteria are basically the ultimate glitch in the matrix and we're just NPCs trying to survive the update? 🤯
Sean Goss
July 24, 2025 AT 19:06The term 'PAMPs' is misused here-should be PRRs recognizing PAMPs. Also, CRISPR is prokaryotic adaptive immunity, not a 'molecular mugshot' system-that's anthropomorphizing beyond utility. This article reads like a BuzzFeed listicle dressed in lab coat jargon.
Khamaile Shakeer
July 26, 2025 AT 14:10Wait… so bacteria are like hackers?? 😱 But like… they’ve been doing this for 3 BILLION years?? And we’re still using hand sanitizer like it’s 2012?? 🤦♂️ #MicrobeWins #BioHacking
Suryakant Godale
July 28, 2025 AT 05:17The mechanistic description of macrophage phagocytosis and dendritic cell antigen presentation is scientifically accurate. However, the anthropomorphic language employed-such as 'sabotage skills' and 'syringe-like tools'-while engaging, risks undermining the rigor expected in scientific communication. A more precise terminology would enhance educational value.
John Kang
July 29, 2025 AT 23:59Honestly this is one of the clearest breakdowns I've seen. You don't need a PhD to get why washing hands matters now. Thanks for making the invisible war feel real.
Bob Stewart
July 31, 2025 AT 02:14The description of horizontal gene transfer as 'collectors trading baseball cards' is imprecise. Horizontal gene transfer involves conjugation, transformation, and transduction-mechanisms governed by plasmids, transposons, and bacteriophages. Analogies should not sacrifice accuracy for accessibility.
Simran Mishra
July 31, 2025 AT 15:15I just read this and I can’t stop thinking about how every single cell in my body is a battlefield and I’m just sitting here scrolling through TikTok like nothing’s happening. I mean, what if one of those little bacteria decides today’s the day they take over? What if I’m already infected and my body’s just pretending everything’s fine? I didn’t sleep last night and now I’m terrified of my own microbiome. I need to go wash my hands again. And maybe cry. And then read more.
ka modesto
August 2, 2025 AT 08:49Love this breakdown! The part about probiotics helping the immune system learn faster? That’s wild. I’ve been eating more kimchi lately and now I feel like I’ve got a tiny army of good guys on my side. 🙌
Holly Lowe
August 2, 2025 AT 13:03Bacteria are the original badasses of evolution. They don’t need LinkedIn profiles or therapy-they just show up, take over, and rewrite the rules. Meanwhile, we’re over here debating whether to get the new vaccine or not. Respect the microbes. They’ve been here since the planet was a hot soup.
Cindy Burgess
August 4, 2025 AT 05:54The article conflates correlation with causation regarding stress and infection susceptibility. While epidemiological data suggest an association, the underlying neuroimmunological pathways require further elucidation before definitive clinical recommendations can be made.
Tressie Mitchell
August 4, 2025 AT 11:52This is undergrad-level pop science dressed as cutting-edge research. CRISPR in bacteria? Please. That’s textbook stuff. And calling Salmonella’s T3SS a 'syringe'? How quaint. If you’re going to write about host-pathogen interactions, at least cite actual Nature papers, not some university press release.
dayana rincon
August 5, 2025 AT 09:32So bacteria are the original influencers. They don’t post selfies… they post infections. 🤷♀️🧬 #MicrobeGang #ImmuneSystemIsOverIt
Orion Rentals
August 6, 2025 AT 15:19The inclusion of clinical case studies from Halifax and Dalhousie University provides valuable geographic and institutional context, enhancing the article’s credibility. The integration of recent findings (2024–2025) demonstrates commendable currency in scientific reporting.
Sondra Johnson
August 6, 2025 AT 21:25I love how this doesn’t paint bacteria as villains-they’re just surviving. Kinda like us. Maybe we should stop trying to annihilate them and start learning how to coexist. Like… maybe we’re the invasive species here? 🤔
Chelsey Gonzales
August 8, 2025 AT 17:35this made me realize i need to stop eating that expired yogurt and maybe sleep more?? like… my body is literally fighting a war and i’m just vibin?? 🤭